T cell reconstitution correlates with outcomes in patients undergoing stem cell transplantation (HCT). A functional thymus is needed for the maturation of T cells, but unfortunately, the thymus is highly sensitive to acute injury, such as cytoreductive conditioning given to HCT patients. To enhance T cell reconstitution, it is therefore imperative to explore therapies that can boost thymic regeneration and T cell output. Regulatory T cells (Tregs) are known to mediate homeostasis and tissue regeneration in a variety of tissues, but their role in thymic injury is not known. This study aims to shed light on the role of regulatory T cells (Tregs) in thymic regeneration.
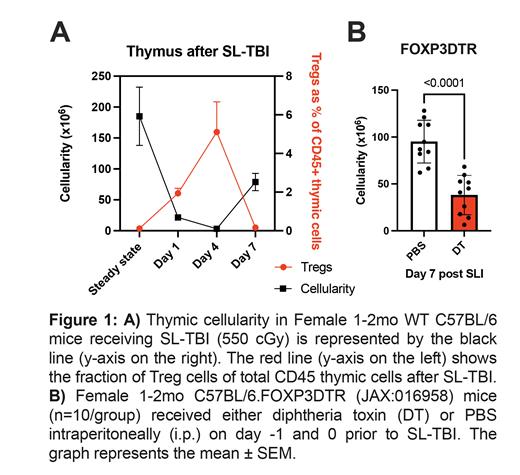
Using a model of thymic injury and regeneration in mice receiving sublethal total body irradiation (SL-TBI, 550cGy), we observed a significant increase in Tregs ( Fig. 1A), located in both the medullary and cortical areas of the thymus on multiparameter immunohistochemistry. Employing a targeted ablation of Tregs at the time of injury using the FOXP3DTR mouse model, we could observe significant delay in thymic regeneration in mice depleted of Tregs (day 7 post SL-TBI, p=<0.0001, n=10, Fig. 1B).
RAG2GFP mice, where GFP is expressed under the RAG2 promoter, have been used to discern recirculating and de-novo produced Tregs. To characterize the nature of the Treg expansion, we treated RAG2GFP mice with SL-TBI and analyzed their thymus at multiple time points after injury. Interestingly, the increase in Tregs was mainly due to recirculating RAG2-GFP- Tregs but not de-novo produced RAG2+GFP+ Tregs after injury. Brdu administration after injury indicated proliferation of these RAG2-GFP- Tregs. Parabiotic experiments linking RAG2GFP mice expressing either the congenic marker CD45.1 or CD45.2 confirmed the recirculating nature of these cells, both at steady state and after injury. Furthermore, when adoptively transferring sorted Thymic Tregs from FOXP3RFP mice to mice receiving SL-TBI, we significantly enhanced thymic cellularity after injury and homing of these Tregs to the thymus.
To discern the transcriptional profile of these cells, we used single-cell sequencing of CD45+ RAG2- cells in 2- and 18-month-old RAG2GFP mice before and at day 1, 4, and 7 after sublethal irradiation. This enabled us to discover the age- and injury-associated changes in recirculating T cells. While the aged thymus had a similar surge of Treg numbers after injury, this response was blunted compared to their young counterparts.
Factor normalization methods enabled us to find two distinct gene regulatory networks highly specific to the RAG2-GFP-Tregs. One gene network was highly enriched for Zfp36l1, a poised RNA known to mediate cell stability. Selectively ablating Zfp36l1 in Tregs using the Foxp3Cre ZFP36L1 flox/flox model impaired regeneration significantly after SL-TBI. The other distinct RAG2-GFP- Treg specific gene network was enriched for the regenerative factor Amphiregulin ( Areg). Amphiregulin was seen to be increased in thymic supernatants, and its receptor Egfr was seen to be transcriptionally upregulated in thymic epithelial cells after injury. In parallel, the most significantly upregulated pathway using cell-to-cell interaction prediction models on single-cell data indicated a strong likelihood of Treg to thymic epithelial cell interactions through amphiregulin to Egfr. Functional inhibition of amphiregulin with an antibody enabled us to observe delayed thymic regeneration, indicating amphiregulin as a factor in the regenerative capacity of thymic recirculating Tregs.
Mapping human gene orthologs of the gene regulatory program provided by factor normalization in murine RAG2GFP- Tregs on single cell data of human pediatric thymic samples from children undergoing open heart surgeries enabled us to distinguish a putatively analogous human population of recirculating thymic Tregs. This population was found to be amphiregulin positive. And human thymic epithelial cell cultures with recombinant amphiregulin enhanced the growth of human thymic epithelial cells, as measured by life imaging, and promoted signalling through MAPK ERK1/2.
Taken together, our findings reveal a novel role for injury-resistant recirculating thymic regulatory T cells existing in both the human and murine thymus and may enable the development of new therapeutic targets for thymic regeneration for patients undergoing cytoreductive therapies.
Disclosures
DeWolf:Atreca: Current equity holder in publicly-traded company, Other: Spouse is an equity holder. van den Brink:Nektar Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Thymofox: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Notch Therapeutics: Consultancy, Current holder of stock options in a privately-held company, Honoraria, Membership on an entity's Board of Directors or advisory committees; Ceramedix: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Lygenesis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pluto Immunotherapeutics: Consultancy, Current holder of stock options in a privately-held company, Honoraria, Membership on an entity's Board of Directors or advisory committees; DKMS (a non-profit organization): Membership on an entity's Board of Directors or advisory committees; Juno Therapeutics: Other: IP licensing; Wolters Kluwer: Patents & Royalties; Vor Biopharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Da Volterra: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Frazier Healthcare Partners: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Rheos Medicines: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Seres Therapeutics: Consultancy, Current holder of stock options in a privately-held company, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: IP licensing , Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal